Heating 235g of water from 22.6C to 94.4C in a microwave oven requires 7.06 x 104J of energy. If the microwave frequency is 2.88 x 105s-1, how many quanta are required to supply the 7.06 x 104J?
You only really need from the question are the frequency of the microwaves (2.88•105s-1), the temperature change (22.6C to 94.4C), and the total energy (7.06•104J). First, we turn the frequency into a wavelength.
λ=c/f
c=299,792,458 m/s
f=2.88•105s-1
λ=299,792,458/(2.88•105) m•s/s
λ=1040.49603
c=299,792,458 m/s
f=2.88•105s-1
λ=299,792,458/(2.88•105) m•s/s
λ=1040.49603
Next, we need to find the temperature change.
T0=22.6°C=295.75K
T1=94.4°C=367.55K
T0=22.6°C=295.75K
T1=94.4°C=367.55K
T=T1-T0
T=367.55-295.75
T=71.8
T=367.55-295.75
T=71.8
Next, we'll need a few constants.
Planck's Constant: h=6.626068•10-34 m2kg/s
Boltzmann's Constant: 1.3806503•10-23 m2•kg•s-2•K-1
e=2.71828183
Now, we're ready to find the energy of one photon, but we need a formula.
Planck's Constant: h=6.626068•10-34 m2kg/s
Boltzmann's Constant: 1.3806503•10-23 m2•kg•s-2•K-1
e=2.71828183
Now, we're ready to find the energy of one photon, but we need a formula.
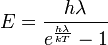
You use all the values in that formula and you get 9.91306903•10-22 J per photon.
Now all you need to do is divide the total energy by the amount of energy each photon caries.
Now all you need to do is divide the total energy by the amount of energy each photon caries.
(7.06•104J)/(9.91306903•10-22J)
7.12191147•1025
7.12191147•1025
So it takes 7.12191147•1025 photons to heat the water, but we need to remember to round to the appropriate number of digits. We started wit 3-digit numbers, so we have to end with a 3-digit number. The final answer is 7.12•1025 photons.
Thanks to Wikipedia for the picture of the formula.
Thanks to Wikipedia for the picture of the formula.
No comments:
Post a Comment